When inflammation goes overboard
A wave of destruction floods organs and tissues
Each year, millions of people in the United States experience trauma, surgery, or infection that causes an undesired overactivation of inflammatory cells and sends the body into shock. Various life support mechanisms, such as continuous renal replacement therapy (CRRT), are employed in an Intensive Care Unit (ICU) setting to save lives and mitigate severe damage to a variety of critical organs such as the heart, lungs, kidney, and liver. In far too many cases, these patients suffer needless loss of life or life-long impairment due to organ damage. Currently, there are no other therapeutic options that specifically and safely neutralize the white blood cells that are primarily responsible for the destructive hyperinflammatory response.
Our first commercial product and lead clinical indication are focused on the treatment of destructive hyperinflammation in patients with acute kidney injury (AKI)
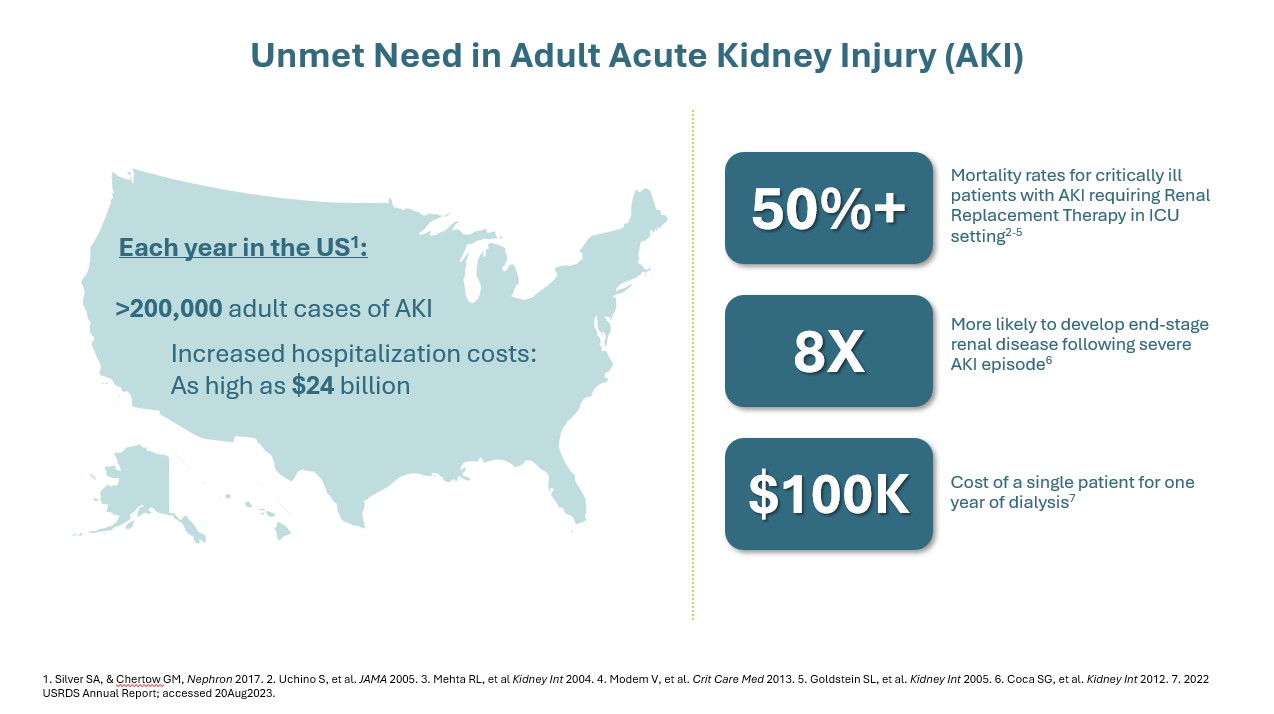
In critically ill patients with AKI, a dysregulated immune response can lead to destructive hyperinflammation. This aggressive response contributes to significant morbidity, including prolonged length of stay in the ICU, increased reliance on dialysis and mechanical ventilation, and increased hospital costs5. Damage resulting from this destructive hyperinflammatory response in AKI can progress to other organs, such as the heart or liver. Multi-organ dysfunction (or even multi-organ failure) can lead to worse outcomes, including cardiovascular events and an increased risk of death6, 7. Even after recovery of the initial insult, these patients may face chronic kidney disease (CKD), end-stage renal disease (ESRD) requiring dialysis, or other complications which can further contribute to increased healthcare utilization costs.8-11
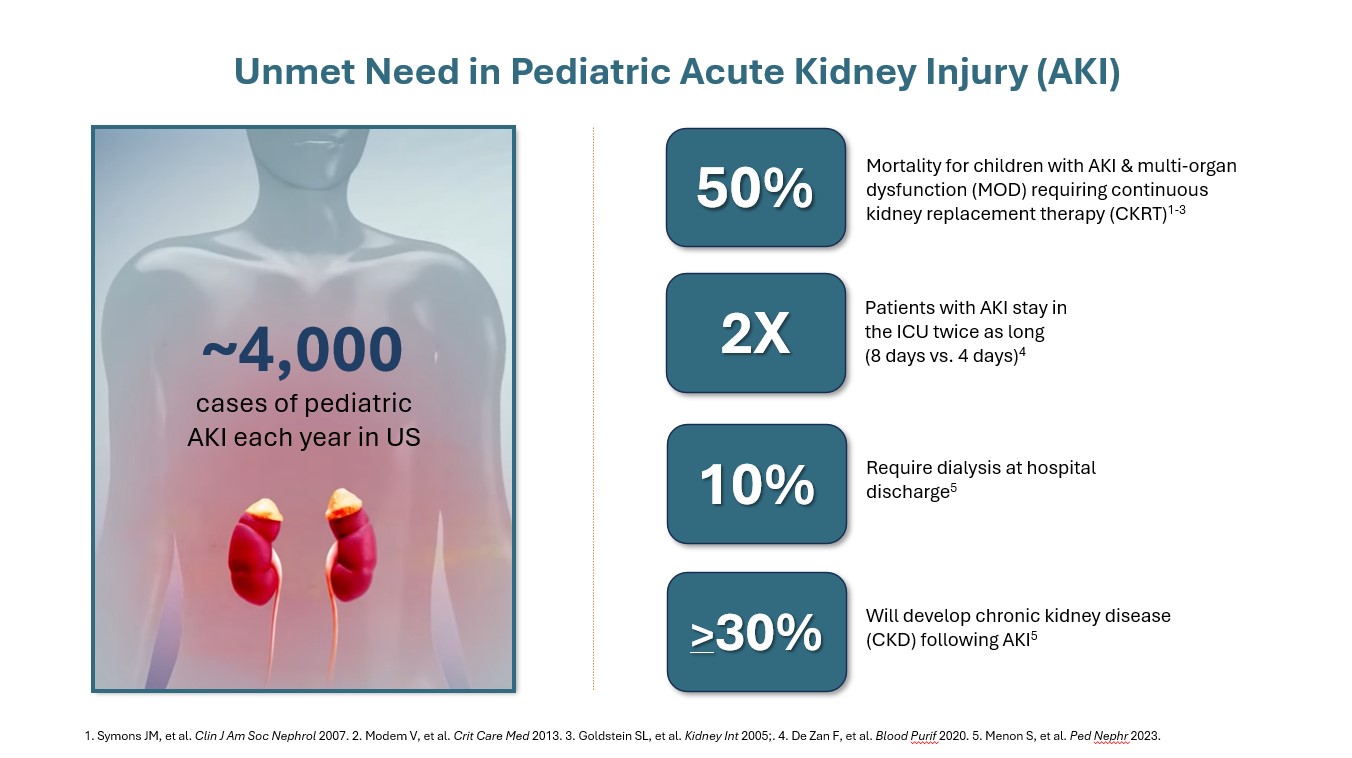
Although the number of pediatric patients who suffer from AKI is far smaller than adults, the impact of this illness in children is still staggering. Tragically, the mortality rate for pediatric patients that suffer from AKI with multi-organ dysfunction requiring CRRT is 50%. Pediatric patients with AKI spend twice as long in the ICU and more than 30% will succumb to chronic kidney disease (CKD) following AKI.
In critically ill patients, stopping destructive hyperinflammation can be crucial to improve outcomes.
References:
1. Malkina A. Acute kidney injury (AKI). Merck Manual Professional Version. 2020. Accessed January 5, 2021.
2. Hassanein M, Radhakrishnan Y, Sedor J, et al. Cleveland Clinic Journal of Medicine. 2020;87(10):619-631.
3. Yap SC, Lee HT. Anesthesiology. 2012;116(5):1139-1148.
4. Hsu RK, Hsu C.Semin Nephrol. 2016;36(4):283-292.
5. Collister D, et al. Clin J Am Soc Nephrol. 2017;12(11):1733-1743.
6. Doyle JF, Forni LG. Crit Care. 2016;20(1):188.
7. Armutcu F. Inflamm Res. 2019;68(10):825-839.
8. Cerda J, et al. Clin J Am Soc Nephrol. 2015;10(10):1859-1867.
9. Godeau E, et al. Case Rep Crit Care. 2019;7953141.
10. Wu VC, et al. J Am Soc Nephrol. 2014;25(3):595-605.
11. Wu VC, et al. J Am Heart Assoc. 2014;3(4):e000933.