INTRODUCING QUELIMMUNE
Now FDA approved for pediatric acute kidney injury (AKI)
due to sepsis or septic conditions!!


What is QUELIMMUNE?
- QUELIMMUNE, also known as the Selective Cytopheretic Device for Pediatrics (SCD-PED), is a humanitarian medical device to treat pediatric patients with acute kidney injury (AKI) due to sepsis or a septic condition. The device is connected in-line to an existing hemodialysis delivery system’s extracorporeal continuous renal replacement therapy (CRRT) circuit.
- QUELIMMUNE was granted approval under a Humanitarian Device Exemption (HDE) by FDA in February 2024 with clinical data establishing safety and probable benefit for this use.
QUELIMMUNE Can Be Efficiently Added to Existing Workflows
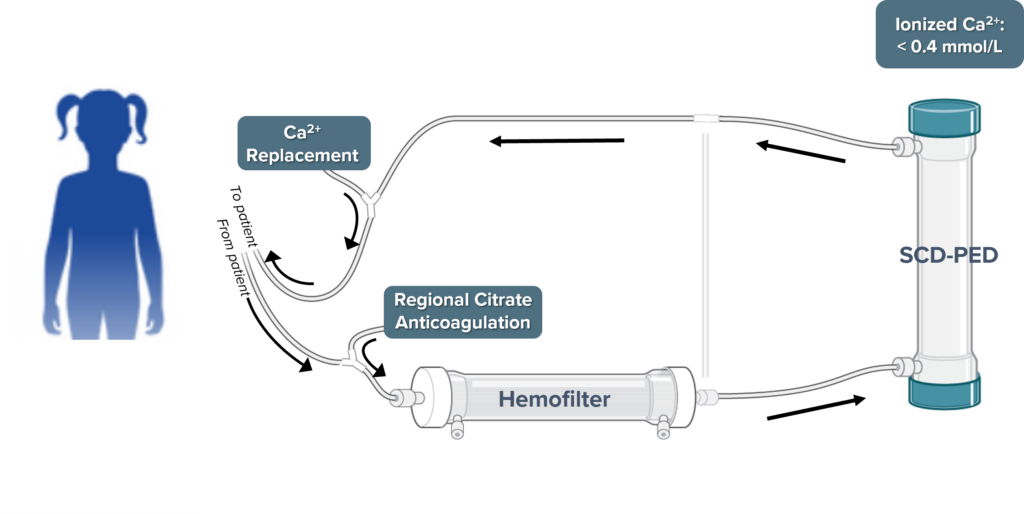
Are you interested in bringing QUELIMMUNE to your hospital?
Eligibility Checklist (to purchase QUELIMMUNE at your hospital):
- Use Prismaflex or PrisMax CRRT machines
- Currently use or agree to use regional citrate anticoagulation (RCA) in the CRRT circuit
- Commitment to participate in the SAVE Surveillance Registry
- IRB approval of SAVE Surveillance Registry
- Institutional resources to complete data collection

Interested in taking the next step?
For more information...
Email us at: quelimmune@seastarmed.com
There are very few therapeutic options available to treat pediatric AKI…these positive [study] results suggest a favorable benefit to risk ratio in the critically ill pediatric population.

INDICATION FOR USE
QUELIMMUNE (SCD-PED) is intended to treat pediatric patients (weight ≥ 10 kg and age ≤ 22 years) with acute kidney injury (AKI) due to sepsis or a septic condition on antibiotic therapy and requiring renal replacement therapy (RRT).
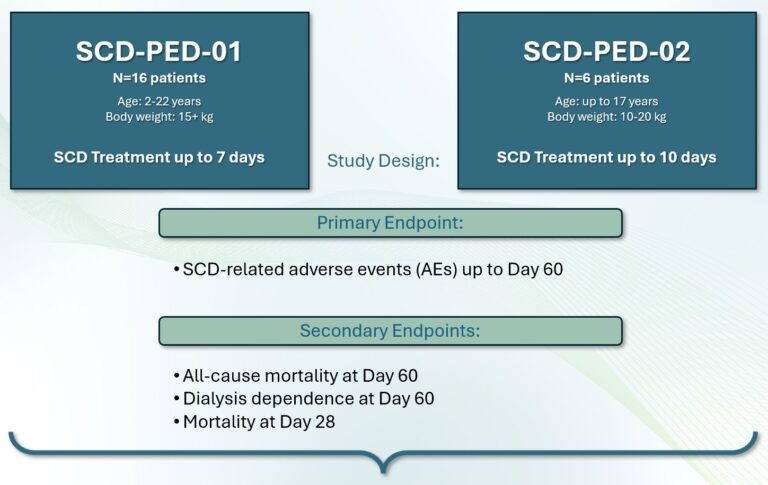
Clinical Studies of the SCD-PED
QUELIMMUNE was evaluated in two multi-center, open-label, prospective clinical trials (SCD-PED-01 & SCD-PED-02) in pediatric intensive care unit (ICU) patients with AKI requiring CRRT.
QUELIMMUNE therapy demonstrated safety & probable benefit in this critically ill population.
Study Design & Endpoints:
Safety Results:

Secondary Outcomes:
QUELIMMUNE (SCD-PED) therapy demonstrated:
– 77% survival rate at Day 60
– No dialysis required in survivors (Day 60 after ICU discharge)
– 87.5% of survivors had normal kidney function (Day 60 after ICU discharge)
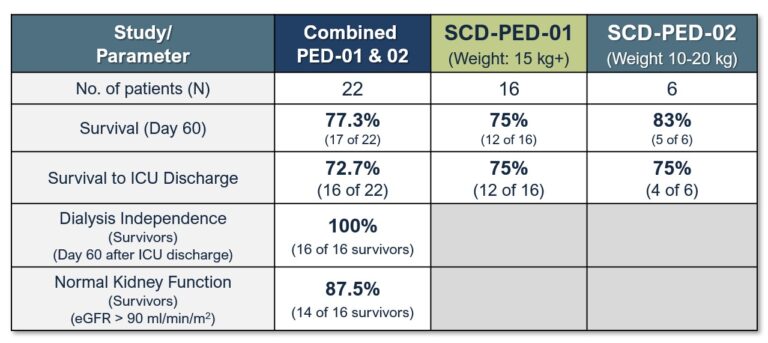
These data were published in the journal Kidney Medicine.
CONTRAINDICATIONS
Use of the SCD-PED as a standalone unit to provide any renal replacement therapy or fluid and electrolyte management therapy is contraindicated.
The SCD-PED cartridge and SCD Blood Tubing Set should not be used on patients who have a known allergy to any components in this product.
ADVERSE REACTIONS
Five adverse reactions were observed in more than one instance across the two pediatric SCD studies (SCD-PED-01 [16 subjects enrolled] and SCD-PED-02 [6 subjects enrolled]). Those adverse reactions include six instances of hypotension across three subjects, four instances of hypothermia across two subjects, four instances of tachycardia across three subjects, two instances of hyperglycemia across two subjects, and two instances of thrombocytopenia across two subjects.
