CHART A NEW COURSE
Groundbreaking therapy transforms destructive hyperinflammation into healing
Devastating outcomes signal an urgent need for innovative solutions
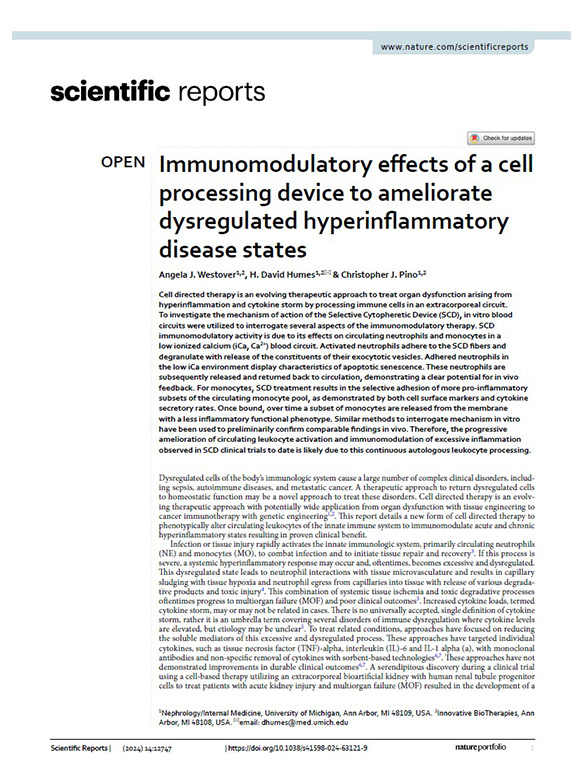
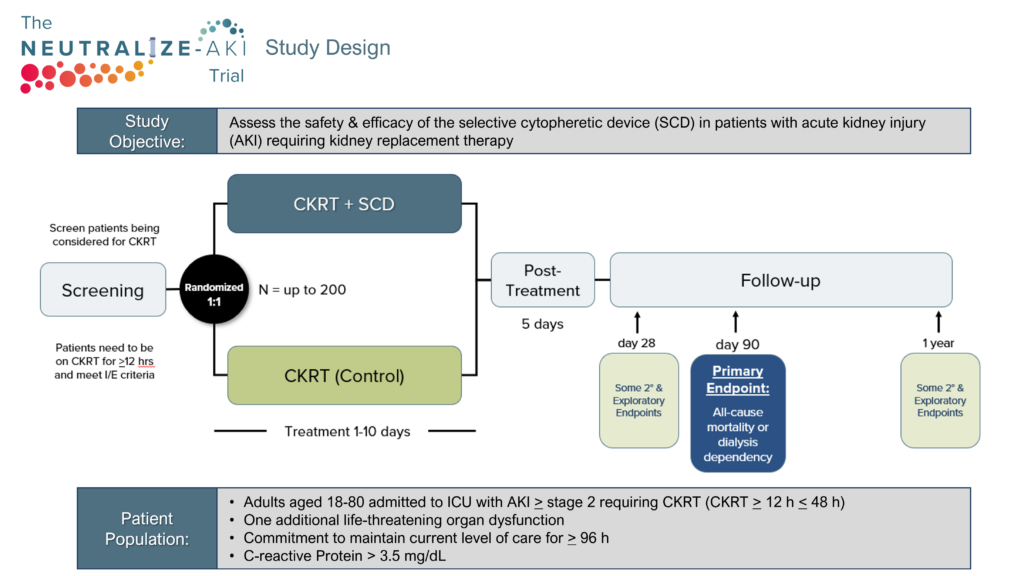
Therapeutic options for critically ill patients experiencing destructive hyperinflammation have shown limited efficacy. Our Selective Cytopheretic Device (SCD) is a patented cell-directed extracorporeal therapy that works with most continuous renal replacement therapy (CRRT) systems to selectively target the most highly activated neutrophils and monocytes responsible for this destructive hyperinflammatory response. The SCD therapy won’t just stop the storm but could potentially reverse the damage.
Neutralize toxic neutrophils and transform monocytes
Neutrophils and monocytes are the key immune cells that play a prominent role in life-threatening hyperinflammation.
The novel SCD technology targets and neutralizes activated neutrophils, providing a new therapeutic approach to this problem.
By restoring reparative physiology, the SCD can help the body heal—potentially reducing mortality, eliminating dialysis dependency and getting patients out of the ICU faster so they can return to their loved ones and daily lives.
Currently, drug treatments for a cytokine storm may differ based on the specific disorder associated with it, and these treatments may not deliver the results your patients need.
Because the SCD is a cell-directed extracorporeal therapy that uses the body’s immune system to heal. You’re in complete control of an effective therapy that can be used without the worry of severe contraindications.
SCD therapy selectively targets highly activated neutrophils and monocytes
An evidence-based breakthrough
SeaStar Medical has been granted Breakthrough Device Designations by the U.S. Food and Drug Administration (FDA) for six different indications related to the treatment of destructive hyperinflammation. The designation is designed to provide timely access to medical devices to speed up development, assessment, and review for FDA approval. SCD therapy was approved in 2024 in pediatric AKI due to sepsis and is currently being investigated in adult AKI and other conditions.
Our approved product
Other indications

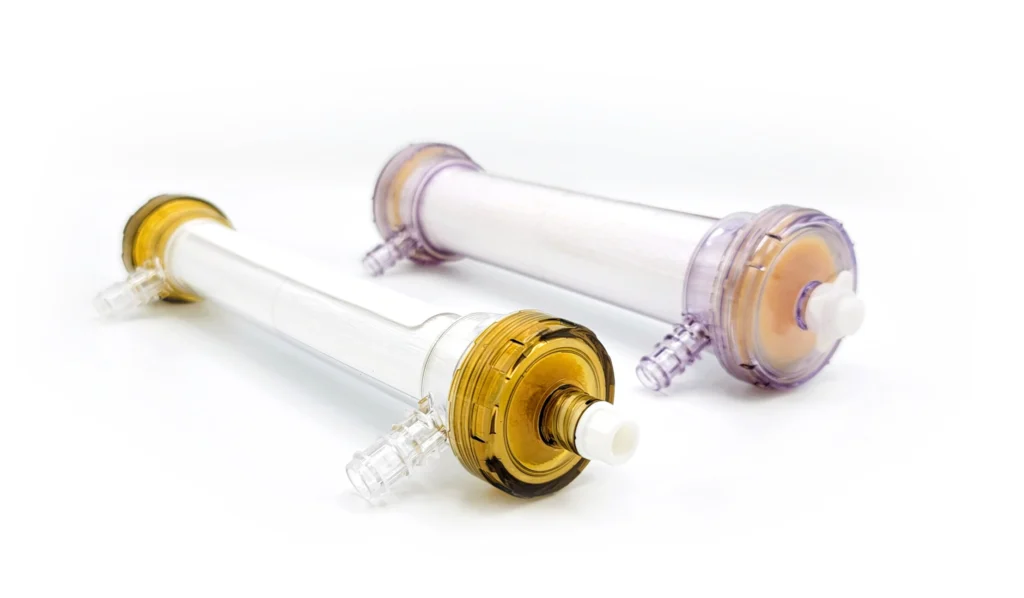
SeaStar Medical is now conducting NEUTRALIZE-AKI to assess the safety and efficacy of the SCD in critically ill adults with AKI requiring CKRT. The study will assess the effect of SCD treatment on various measures of patient clinical outcomes and evaluate the effect of SCD device on patient safety in SCD treatments from time of initiation through one year.
Primary endpoint:
A composite of all-cause mortality or dialysis dependency at day 90.
Secondary endpoints include:
- Major Adverse Kidney Events at day 90 (MAKE90)
- Dialysis dependence at 1 year
- ICU free days in the first 28 days
- Mortality at day 28
For more information, visit www.clinicaltrials.gov.

Clinical studies of the SCD-PED
SCD-PED therapy has been evaluated in two open-label studies (SCD-PED-01 and SCD-PED-02) in pediatric patients >10kg with AKI requiring continuous kidney replacement therapy (CKRT).
Primary endpoint:
Safety
Secondary endpoints:
Mortality and renal replacement therapy dependency at Day 60
Number of participants:
N=22 (both studies combined)
Results:
- No device-related adverse events, infections or immunosuppression
- 77% survival rate (Day 60)
- 100% renal recovery rate at 60 days in surviving children
Easy to incorporate into the ICU workflow and treatment paradigm
The SCD therapy can be easily added to extracorporeal therapies, such as dialysis or continuous renal replacement therapy (CRRT), seamlessly fitting into your workflow. It has been used with other treatments, including IL-6 blockers, corticosteroids and other common treatments, with no or few known contraindications.
By giving critically ill patients the potential to eliminate dialysis dependency, get out of the ICU faster, and restore the lives they were so close to losing, the SCD is positioned to become the new standard of care in the ICU.
SCD in the Medical Literature
Get in touch with SeaStar Medical
Intrigued by the promise of the SCD? Want to know more? We’ll be happy to hear from you.