When inflammation goes overboaRd
Insights into the life-threatening cytokine storm
Defining the cytokine storm
Cytokines—small, cell-signaling proteins—are integral in the immune system’s armamentarium, regulating immune and inflammatory responses at the site of injury or infection.1 But in many critical-care patients, a dysregulated cytokine response can cause hyperinflammation, known as a cytokine storm, and this can lead to tragic outcomes unless stopped in time.

The genesis of the cytokine storm
Inflammatory cytokines respond to injury or infection by increasing local blood flow and temperature and mobilizing immune cells to the site.1,2 Neutrophils and monocytes—leukocytes (white blood cells) that are integral to the inflammatory process—produce cytokines and are also recruited to an injured or infected site.3,4
In a normal immune response, neutrophils are the first immune cells to arrive at the site and are key to the entire immune response through a variety of functions, including the production of neutrophil extracellular traps (NETs) that kill pathogens.3-6 Because neutrophils and monocytes have heterogenic properties, they are also integral to tissue remodeling and repair.7
In a dysregulated immune response, overactive monocytes and NETs lead to the overproduction of cytokines,4,7,8 and neutrophil apoptosis may be delayed.3 Additionally, feedback mechanisms that regulate the immune system are altered, so negative feedback is all but absent while positive feedback is left unchecked, with cytokines continually recruiting immune cells.9 This results in damaging hyperinflammation at the site, but it doesn’t stop there.
The destructive path of the cytokine storm
In the cytokine storm, local hyperinflammation spreads uncontrollably through the systemic circulation to other parts of the body, leading to endothelial dysfunction and consequent organ damage.1 Without effective treatment, patients can suffer from catastrophic organ failure and lose their lives.
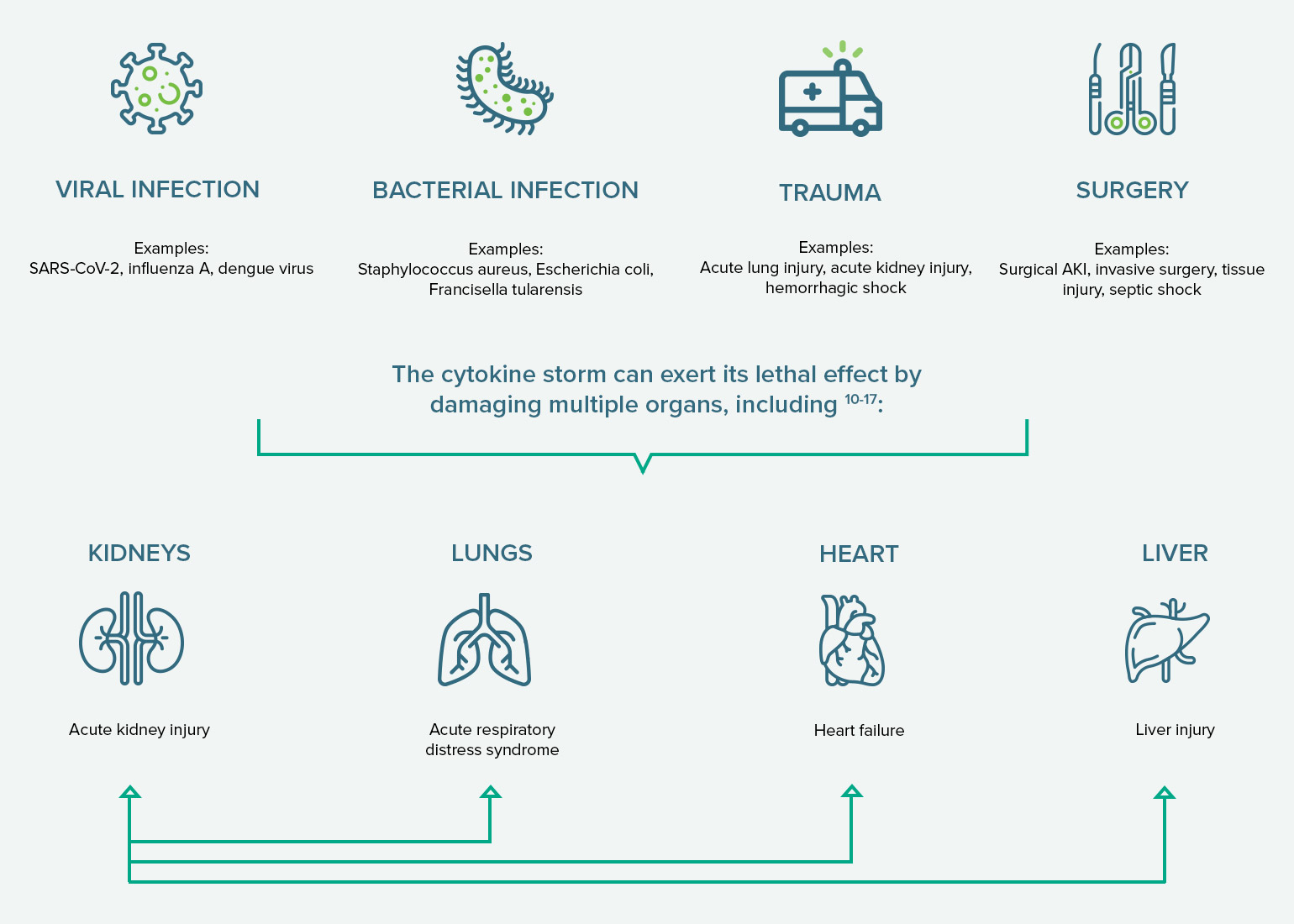
Pathogenic and mechanical triggers of a cytokine storm
VIRAL INFECTION
Examples:
SARS-CoV-2, influenza A, dengue virus
BACTERIAL INFECTION
Examples:
Staphylococcus aureus, Escherichia coli, Francisella tularensis
TRAUMA
Examples:
Acute lung injury, acute kidney injury, hemorrhagic shock
SURGERY
Examples:
Surgical AKI, invasive surgery, tissue injury, septic shock
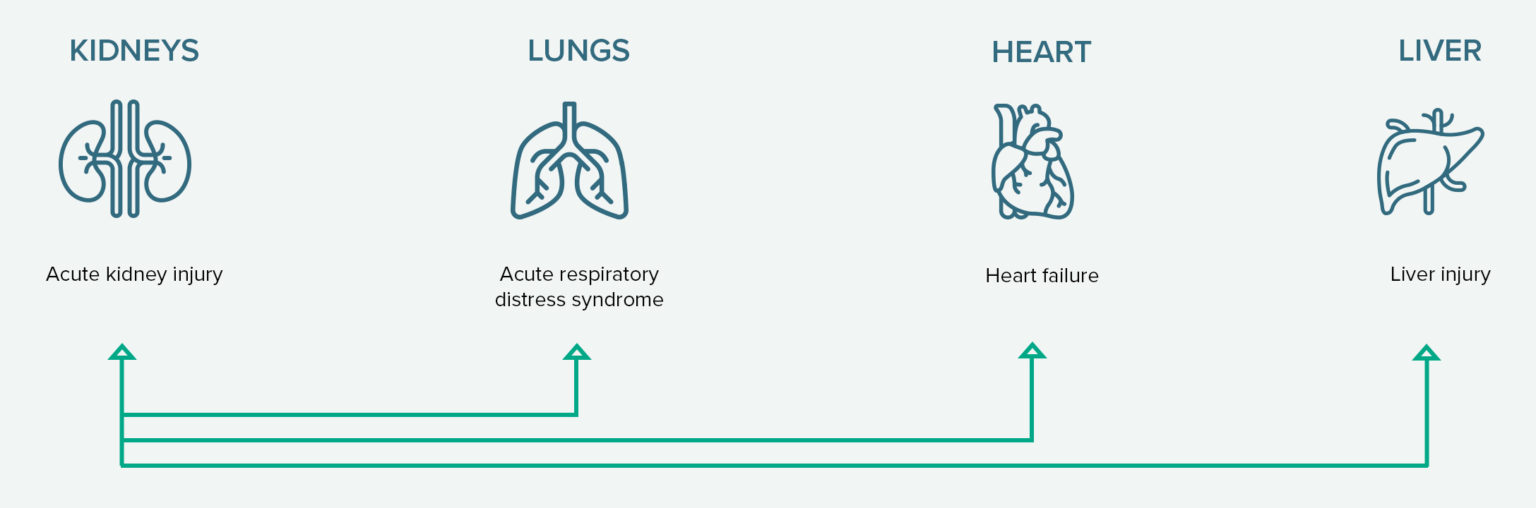
Through organ crosstalk, damaged organs can cause further destruction to other organs (as illustrated by arrows). Note: These are not all the possible morbidities associated with the cytokine storm.
Impact of Cytokines on the Kidneys and Lungs
The COVID-19 pandemic has raised understanding of the connection between the lungs and the kidneys. Loss of normal function in either organ can induce direct and indirect dysregulation of the other, as seen with COVID-19 patients.
There are two phases of COVID-19: the viral replicative phase and the hyperinflammatory phase. The latter is the most severe phase of the disease, manifesting as extra-pulmonary systemic hyperinflammation syndrome.18 Coupled with the disease’s inhibition of the adaptive immune response—including a reduction in helper, suppressor, and regulatory T cell counts—the cytokine storm can make COVID-19 deadly.12,18
COVID-19-associated AKI and ARDS are prevalent and deadly:
In studies of hospitalized COVID-19 patients:
with a 50% mortality rate19
Among hospitalized COVID-19 patients with AKI, 19% required dialysis.19
with a 45% mortality rate20
Approximately 75% of COVID-19 patients in the ICU had ARDS.20
AKI
- AKI—a sudden and temporary loss of kidney function—can be caused by a wide range of conditions, such as COVID-19, sepsis, severe trauma, and surgery and can lead to end stage renal disease21-24
- AKI is responsible for inflammatory and oxidative stress mechanisms that damage multiple other organs, including the lungs, heart, liver, intestines, and brain 23
- Inflammatory cytokines are among the biomarkers implicated in high morbidity and mortality in patients with AKI25
ARDS
- ARDS can be precipitated by a hyperinflammatory response to a wide range of lung insults, including SARS-CoV-2, other pathogens, sepsis, pneumonia, aspiration, and inhalation injury1,26
- Renal involvement in chronic respiratory diseases is commonly observed in clinical practice. Several studies have reported that prevalence of renal failure is higher in patients with diseases affecting mainly the lungs.
- An international study with over 29,000 patients found that 10% of ICU admissions and 23% of ventilation cases were due to ARDS27
In critically ill patients, stopping the cytokine storm can be crucial to improve outcomes.
References: 1. Tisoncik JR, Korth MJ, Simmons CP, et al. Into the eye of the cytokine storm. Microbiology and Molecular Biology Reviews. 2012;76(1):16-32.
2. Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediators of Inflammation. 2013;2013:1-16. http://dx.doi.org/10.1155/2013/165974.
3. Bordon J, Aliberti S, Fernandez-Botran R, et al. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. International Journal of Infectious Diseases. 2013;17:e76-e83. http://dx.doi.org/10.1016/j.ijid.2012.06.006.
4. Barnes BJ, Adrover JM, Baxter-Stolzfus A, et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6):1-7. https://doi.org/10.1084/jem.20200652.
5. Eberl M, Davey M. Neutrophils. BiteSized Immunology. British Society for Immunology. https://www.immunology.org/public-information/bitesized-immunology/cells/neutrophils. Accessed November 27, 2020.
6. Delgado-Rizo V, Martinez-Guzman MA, Iñiguez-Gutierrez L, et al. Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol. 2017;8:1-20. doi: 10.3389/fimmu.2017.00081.
7. Rao X, Zhong J, Sun Q. The heterogenic properties of monocytes/macrophages and neutrophils in inflammatory response in diabetes. Life Sci. 2014;116(2):59-66. doi:10.1016/j.lfs.2014.09.015.
8. Tomar B, Anders H-J, Desai J, et al. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020;9(1383):2-8. doi:10.3390/cells9061383.
9. Song P, Li W, Xie J, et al. Cytokine storm induced by SARS-CoV-2. Clinica Chimica Acta. 2020;509:280-287. https://doi.org/10.1016/j.cca.2020.06.017.
10. Mustafa MI, Abdelmoneim AH, Mahmoud EM, et al. Cytokine storm in COVID-19 patients, its impact on organs and potential treatment by QTY code-designed detergent-free chemokine receptors. Mediators of Inflammation. 2020. https://doi.org/10.1155/2020/8198963.
11. Effenberger M, Grander C, Grabherr F, et al. Systemic inflammation as fuel for acute liver injury in COVID-19. Digestive and Liver Disease. 2020. https://doi.org/10.1016/j.dld.2020.08.004.
12. Wang M, Xiong H, Chen H, et al. Renal injury by SARS-CoV-2 infection: a systematic review. Kidney Dis. 2020. doi: 10.1159/000512683.
13. WebMD. What causes a cytokine storm? https://www.webmd.com/lung/qa/what-causes-a-cytokine-storm. Accessed November 25, 2020.
14. Johns Hopkins Medicine: Health. Can coronavirus cause heart damage? https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/can-coronavirus-cause-heart-damage. Accessed November 25, 2020.
15. Go AS, Hsu C, Yang J, et al. Acute kidney injury and risk of heart failure and atherosclerotic events. Clin J Am Soc Nephrol. 2018;13:833-841. https://doi.org/10.2215/CJN.12591117.
16. Batlle D, Soler MJ, Sparks MA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. JASN. 2020:1380-1383. https://doi.org/10.1681/ASN.2020040419.
17. Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet. 2020(8):738-742. https://doi.org/10.1016/S2213-2600(20)30229-0.
18. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical−therapeutic staging proposal. Journal of Heart and Lung Transplantation. 2020;39(5):405-407. https://doi.org/10.1016/j.healun.2020.03.012.
19. Chan L, Chaudhary K. Saha A, et al. AKI in hospitalized patients with COVID-19. JASN. 2020. https://doi.org/10.1681/ASN.2020050615.
20. Tzotzos SJ, Fischer B, Fischer H, et al. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Critical Care. 2020;24;516. https://doi.org/10.1186/s13054-020-03240-7.
21. Malkina A. Acute kidney injury (AKI). Merck Manual Professional Version. 2020. https://www.merckmanuals.com/professional/genitourinary-disorders/acute-kidney-injury/acute-kidney-injury-aki. Accessed January 5, 2021.
22. Hassanein M, Radhakrishnan Y, Sedor J, et al. COVID-19 and the kidney. Cleveland Clinic Journal of Medicine. 2020;87(10):619-631. doi:10.3949/ccjm.87a.20072.
23. Yap SC, Lee HT. Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology. 2012;116(5):1139-1148.
24. Hsu RK, Hsu C. The role of acute kidney injury in chronic kidney disease. Semin Nephrol. 2016;36(4):283-292. doi:10.1016/j.semnephrol.2016.05.005.
25. Takkavatakarn K, Susantitaphong P, Eiam-Ong S. Hemodiafiltration in acute kidney injury. InTechOpen. 2018. doi: 10.5772/intechopen.79563.
26. Jain R, DalNogare A. Pharmacological therapy in acute respiratory distress syndrome. Mayo Clin Proc. 2006;81(2):205-212.
27. Papazian L, Aubron C, Brochard L, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69. https://doi.org/10.1186/s13613-019-0540-9.