pIPELINE
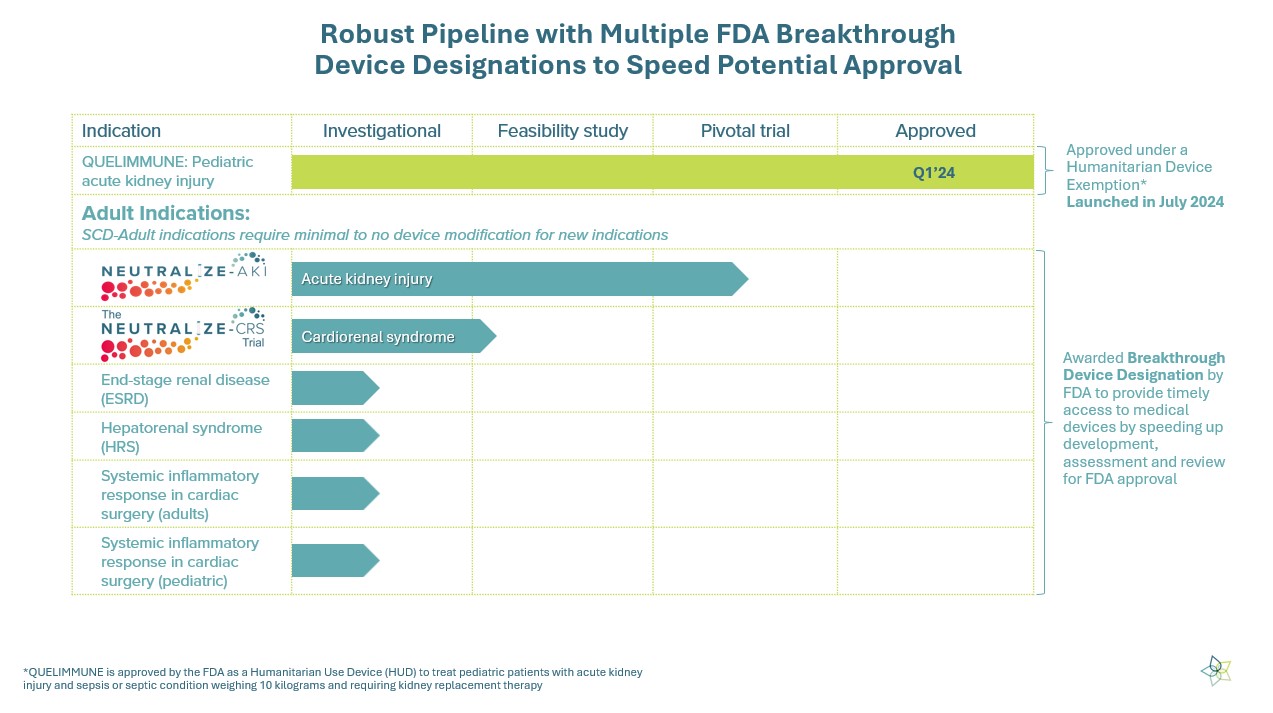
FDA has granted Breakthrough Device Designations for most of our pipeline products
We have a robust pipeline of indications that represent life-threatening or irreversibly debilitating diseases or conditions. These indications have been granted Breakthrough Device Designation by the FDA, which is designed to speed up development, assessment, and review for FDA approval.
Adult AKI and CRRT

Each year in the US, approximately 210,000 adult patients suffer from acute kidney injury (AKI) that can lead to destructive hyperinflammation, causing organ failure and loss of life. We are conducting the NEUTRALIZE-AKI pivotal trial to assess the safety & efficacy of the Selective Cytopheretic Device (SCD) therapy in 200 critically ill adults with AKI in the ICU requiring continuous renal replacement therapy (CRRT). The trial will evaluate approximately 100 patients treated with SCD in addition to CRRT as the standard of care, compared with the control group of approximately 100 patients receiving only CRRT standard of care. The trial will also include subgroup analyses to explore the effectiveness of SCD therapy in AKI patients with sepsis and acute respiratory distress syndrome.
Primary endpoint: A composite of all-cause mortality or dialysis dependency at day 90.
Secondary endpoints include:
- Major Adverse Kidney Events at day 90 (MAKE90)
- Dialysis dependence at 1 year
- ICU free days in the first 28 days
- Mortality at day 28
For more information, visit www.clinicaltrials.gov (ID #: NCT05758077)
Read more about the clinical trial design here.
Cardiorenal Syndrome (CRS)

Cardiorenal syndrome (CRS) is a clinical disorder in which therapy to relieve the congestive symptoms of chronic heart failure is limited by a decline in renal function. We estimate that each year in the US approximately 60,000 patients with cardiorenal syndrome and are awaiting left ventricular assist device (LVAD) implantation.
A feasibility study is underway to evaluate our SCD therapy for the reduction of inflammation in adult patients with acute heart failure with worsening renal function due to cardiorenal syndrome or severe right ventricular failure awaiting a LVAD implantation.
The study is expected to enroll 20 patients at up to five clinical sites and will be funded by a $3.6 million National Institutes of Health (NIH) grant. H. David Humes, MD, Professor, Division of Nephrology, Internal Medicine, University of Michigan and SeaStar Medical Scientific Advisor, will serve as lead investigator for the study, and we will act as clinical research organization (CRO).
The FDA granted Breakthrough Device Designation for the SCD in cardiorenal syndrome with LVAD in September 2023. In addition to our submission of preclinical data, our submission included a first-in-human study of a patient with severe chronic heart failure (CHF) who was ineligible for heart transplantation or LVAD implantation. The patient was treated with our SCD therapy and achieved the primary endpoint of a successful LVAD implantation. Following the surgery, the patient was discharged to home. Additionally, the procedure was safe and there were no SCD-related serious adverse events (SAEs). Read more here.
Hepatorenal Syndrome (HRS)
Each year in the US, an estimated 50,000 patients are impacted by hepatorenal syndrome, a condition that results in abrupt deterioration of kidney function, driven by a hyperinflammatory process in patients with advanced liver cirrhosis, and is associated with an unacceptably high mortality.
An investigator-initiated pilot study conducted at the University of Michigan assessed treatment with the SCD in two patients with type 1 hepatorenal syndrome. Positive clinical outcomes were seen in both cases – one patient with hepatorenal syndrome due to acute alcoholic hepatitis was alive at day 90 after seven days of SCD treatment and undergoing liver transplantation evaluation, and the other patient with hepatorenal syndrome due to non-alcoholic steatohepatitis or NASH had a successful liver transplantation 6 days after SCD therapy ended. This suggested a role of SCD immunomodulation to treat acute on chronic liver failure, regardless of the etiology, as a bridge to evaluation or successful intervention for liver transplantation. Both of these cases were recently published in the American Society for Artificial Internal Organs journal in August 2023.
In October 2023, FDA granted Breakthrough Device Designation for SCD therapy for use in ICU patients with AKI and acute on chronic liver failure. This represents the third Breakthrough Device Designation granted by FDA to SeaStar Medical for the SCD device.
Chronic Systemic Inflammation in ESRD Patients on Chronic Hemodialysis
Patients with end-stage renal disease (ESRD) face an unacceptably high disease burden, including chronic fatigue, malnutrition, repeat hospitalizations, and a 42% 5-year survival. Systemic inflammation is a major driver that leads to these poor outcomes. Approximately 500,000 people in the US with ESRD are treated by in-center hemodialysis at least three times per week at a cost of approximately $100,000 per-patient per-year. We estimate that each year approximately 80,000 of patients on chronic dialysis also incur chronic systemic inflammation that is associated with increased mortality from cardiovascular disease, infections and reduced quality of life.
Initial data shows that the SCD has the potential to address chronic systemic inflammation that could improve clinical outcomes. In November 2024, FDA granted Breakthrough Device Designation for the SCD therapy to treat chronic systemic inflammation in ESRD patients who require chronic hemodialysis. This represents the first Breakthrough designation for the SCD therapy in a chronic indication.
Systemic Inflammatory Response in Patients Undergoing Cardiac Surgery
Patients undergoing cardiopulmonary bypass (CPB) during heart surgery are subject to hyperinflammation with downstream clinical complications that impact multiple organs such as the kidneys, heart, brain, and lungs, among others. Approximately 15% of the estimated 300,000 adults that undergo cardiac surgery each year are considered high risk and, we believe, could benefit most from the SCD therapy to prevent post-surgical complications. Of the 40,000 pediatric patients that undergo congenital heart surgery each year, we believe that approximately 1/3 might benefit from SCD therapy.
Initial data show that SCD therapy has the potential to treat those most unfortunate patients that today undergo lifesaving surgery, only to succumb to the consequences of uncontrolled inflammation.
In April 2025, FDA granted Breakthrough Device Designation for SCD therapy to treat systemic inflammatory response in adult patients undergoing cardiac surgery and also granted the designation to SeaStar Medical’s first pediatric indication for pediatric patients undergoing cardiac surgery towards prevention of post-operative adverse complications and outcomes.