Transforming the destructive immune response, sparing organs and saving lives
When trauma or infection causes the immune system to overreact, destructive hyperinflammation can give rise to a life-threatening cytokine storm, resulting in organ failure, and tragically, loss of life. We are on a mission to save millions of lives by calming the cytokine storm and restoring immune system balance.

transformational
Patented technology that transforms key inflammatory messengers from agents of destruction to agents of repair
disease modifying
Designed to alter patients’
disease progression
seamless integration
Integrates seamlessly into existing hemodialysis delivery systems
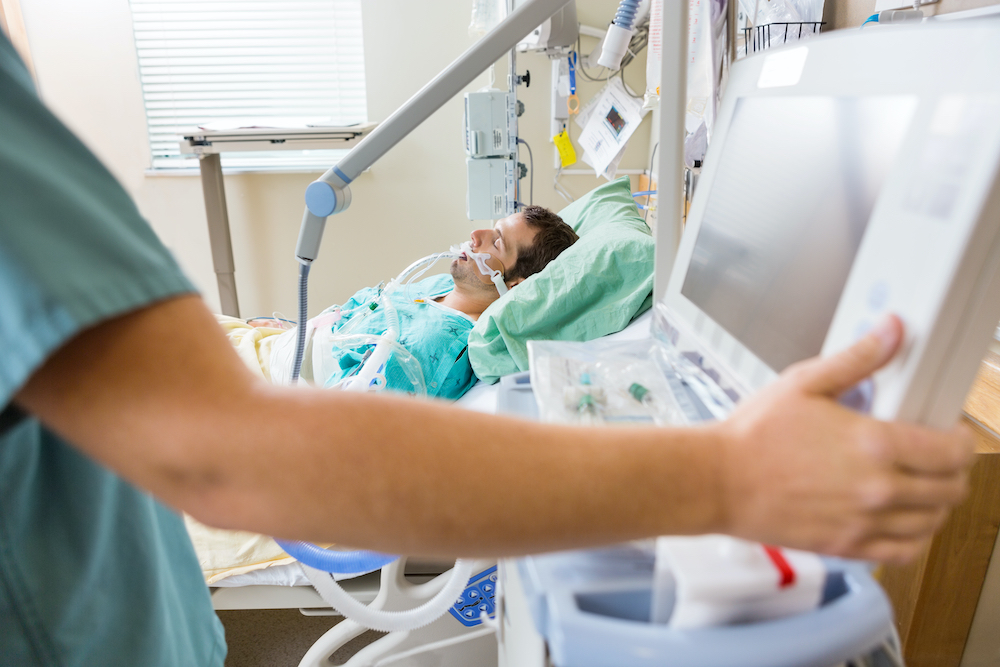
An alarmingly urgent situation
Critically ill patients in the ICU often face an alarming, urgent situation. A dangerous, dysregulated hyperinflammatory response to infection, trauma or surgery can lead to multiorgan failure and in severe cases, even death. Today, there are no other FDA-approved therapeutic options to calm destructive hyperinflammation and restore balance in these critically ill patients.
SeaStar Medical is an innovator with groundbreaking solutions that are designed to stop this serious threat before it can do irreparable harm to these critically ill patients.
Backed by robust scientific evidence, our patented Selective Cytopheretic Device (SCD) is designed to neutralize the hyperinflammatory response to improve outcomes and save precious lives.
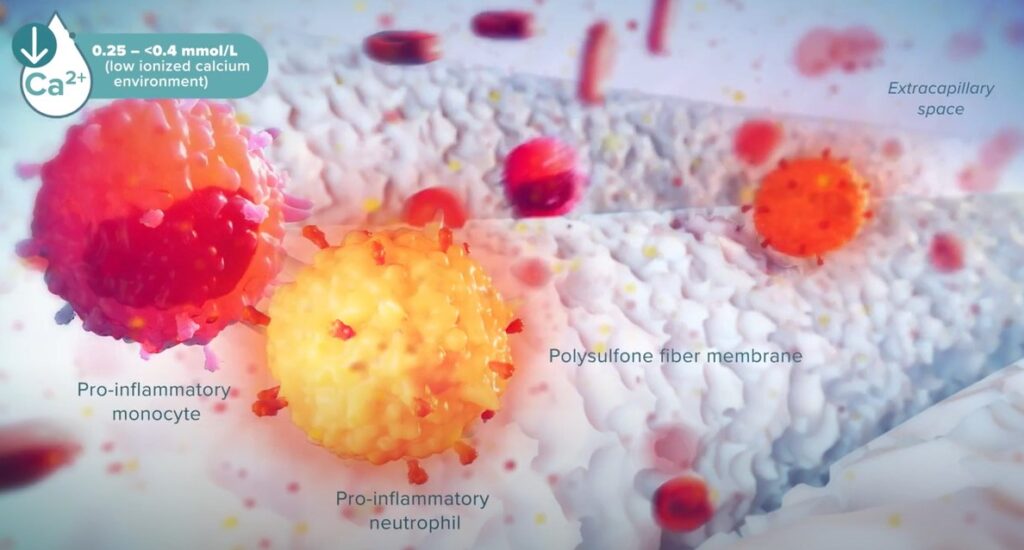
The game-changing selective cytopheretic device (SCD)
Our patented SCD therapy selectively targets the most highly activated neutrophils and monocytes responsible for destructive immune responses. The SCD technology not only stops the cytokine storm but may also reverse the damage of destructive hyperinflammation.

Better outcomes
A shorter treatment regimen to reach the desired effect may reduce dependency on dialysis and time in the ICU. This can help put patients on the road back to recovery sooner and free up resources more quickly.