Making waves.
Changing outcomes.
With our simple approach to addressing a key source of a dysregulated immune response, SeaStar Medical is advancing the science of cell-directed extracorporeal therapy to prevent the devastation of hyperinflammation
chart a new course
Patented technology that transforms key inflammatory messengers from agents of destruction to agents of repair
Improve patient
outcomes
Enables you to alter patients’ disease progression and put them on the path to healing
Keep workflow
on track
Seamlessly integrates into your critical-care workflow
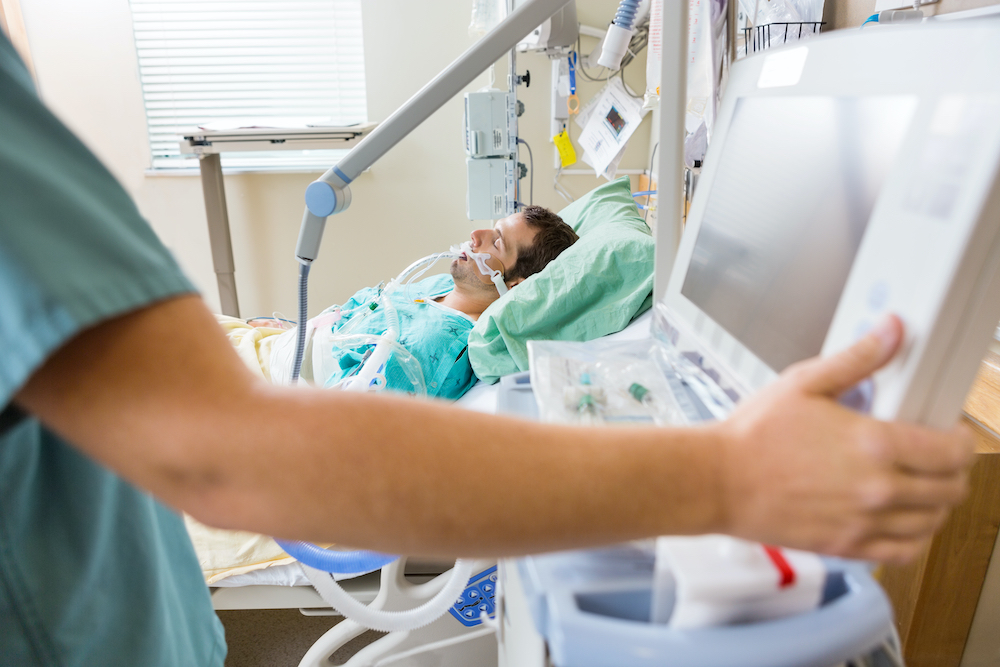
Giving you the power to defeat a serious clinical threat
Critically ill patients in the throes of a cytokine storm are facing an alarming, urgent situation. That’s because this dangerous, dysregulated hyperinflammatory response can cause multiorgan damage and in severe cases, death.
SeaStar Medical is an innovator with groundbreaking solutions that give you the power to stop this serious threat before it can do irreparable harm to the patients you are fighting for.
Backed by rigorous clinical research and robust scientific evidence, our patented approach is designed to support your clinical end goals including reducing dialysis dependency, improving outcomes, and saving precious lives.
Introducing the game-changing Selective Cytopheretic Device (SCD)
Hyperinflammation, or the cytokine storm, develops when the immune system responds too aggressively to injury or infection. Acute kidney injury, acute respiratory distress syndrome, COVID-19, and many other health issues can result in a dangerous cytokine storm. With SeaStar Medical’s novel, simple-to-use SCD cell-directed extracorporeal therapy, you can quickly rescue patients from a cytokine storm, regardless of how it was triggered, and restore reparative functionality to the body and potentially reverse injury.

The SCD is an investigational device and is not currently available for sale. Please check back for further updates on device availability in your region.
Image shown for illustration only and is subject to change.

Better outcomes
A shorter treatment regimen to reach the desired effect may reduce dependency on dialysis and time in the ICU. This can help put patients on the road back to recovery sooner and free up resources more quickly.